The Properties and Reactions of Alkanes
Alkanes are saturated hydrocarbons. They contain only carbon - carbon single bonds and carbon - hydrogen single bonds. They are usually used as fuels and lubricants but are also often used as starting materials for other more complicated products.
The general formula for all chain alkanes is CnH2n+2.
Unbranched Alkanes
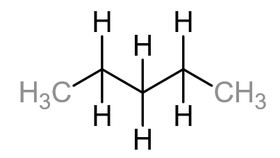
Unbranched alkanes are sometimes called straight chain alkanes. This however is not entirely true as the bond angle of the carbon – carbon – carbon angle is 109.5 which gives it a more zigzag appearance. Below is a displayed formula of pentane:
Branched Alkanes
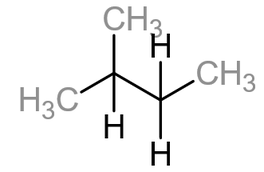
This is when the main chain has another carbon chain coming off from it. These are known as branches. Below is the displayed formula of methylbutane, which is an isomer of pentane:
Ring Alkanes
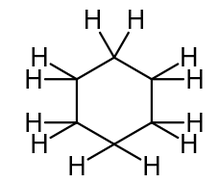
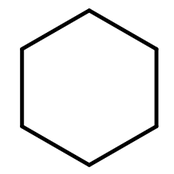
These have the general formula CnH2n. This is because there are no ‘end’ hydrogens, so there is no need for the ‘+2’ in the general formula. Below is the displayed formula AND skeletal formula of cyclohexane:
Physical Properties
Alkanes are almost non-polar due to the relative similarities between the electronegativities of hydrogen and carbon. The only intermolecular forces between the molecules are the weak “Van der Waals forces” though, the larger the molecule, the stronger these become.
As the chain length (numbers of carbons) increases, the melting and boiling points of the alkanes gradually increase for these compounds. The shorter chains are gases at room temperature, pentane with five carbons is a liquid at room temperature though has a very low boiling point. Once we reach about 18 carbons the alkanes become solids at room temperature and have a waxy feel.
Branched alkanes have a lower boiling point than straight chain alkanes. This is because they cannot pack together very well, and this limits the number and effectiveness of the intermolecular forces.
Alkanes in general are quite unreactive. Their strong, non-polar bonds make them unreactive to electrophiles, nucleophiles, acids, bases, oxidising or reducing agents. But they do burn and they will react with halogens under the right conditions.
Reactions of Alkanes
Combustion
Short chain alkanes burn completely in a good supply of oxygen to give carbon dioxide and water:
CH4(g) + 2O2(g) --> CO2 (g) + 2H2O(l) ΔH = -890 kJ mol-1
These combustion reactions give out large amounts of heat and have a very high negative enthalpy value, making them incredibly important to us as fuels. Examples: Methane (natural gas), Propane (camping gas), Butane (calor gas), petrol (a mixture, averaging 8 carbons in length) and paraffin (a mixture between 10-18 carbon in length)
Incomplete combustion
In a limited supply of oxygen, the poisonous gas carbon monoxide, CO is formed, for example:
C3H8 (g) + 3½O2 (g) --> 3CO (g) + 4H2O (l)
This is called incomplete combustion. With even less oxygen, carbon (soot) is produced. This often happens with longer chain hydrocarbons, which need more oxygen to burn compared with shorter chains.
Reaction with halogens
A halogen and an alkane will react if a bright light source is present. It is the UV component of the light which starts the reaction. Alkanes do not react with halogens in a dark room at room temperature (except for fluorine which will react in both the dark and the cold!). The mechanism for these reactions is known as free radical substitution, if you wish to know more, check out its dedicated page in the menu.
C6H14(g) + Br2(l) --> C6H13Br(l) + HBr(g)