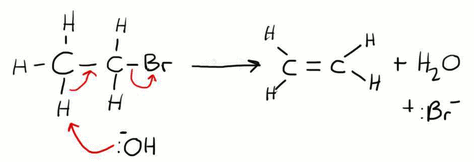Haloalkane Mechanisms
Forming Alkenes from Haloalkanes
Click image to see animation
This is an example of an elimination reaction. Here the OH- ion is acting as a base rather than a nucleophile, meaning that it is accepting a proton. It removes the H from the carbon that is alpha to the C which contains the bromine atom (alpha simply means next door to). For this to work the reaction needs to be in ethanol with NO water present, this can also be described as anhydrous or ethanolic.
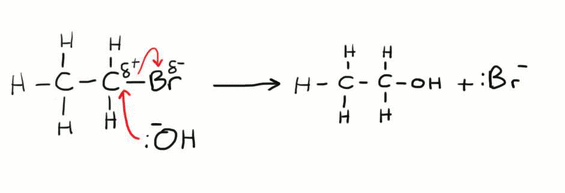
Forming Alcohols from Haloalkanes
Click image to see animation
This is an example of a nucleophilic substitution reaction. The OH- ion is acting as a nucleophile by donating its pair of electrons to the delta positive carbon atom. The carbon is slightly positive due to the electronegativity difference between it and the bromine atom. This reaction can also be described as a hydrolysis reaction. For this to work it needs to be in a warm aqueous solution of sodium hydroxide.
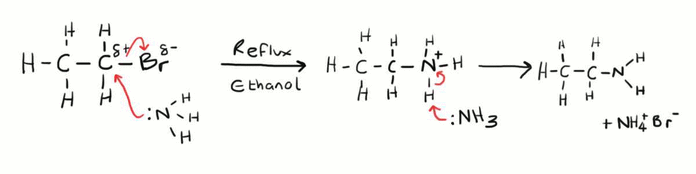
Forming Amines from Haloalkanes
Click image to see animation
This is another example of a nucleophilic substitution reaction, but this time the nucleophile is ammonia (NH3). The ammonia nucleophile donates its pair of electrons to the delta positive carbon forming a bond. This means that the carbon bromine bond is then broken, and the electron pair in the covalent bond is given to the bromine atom. However the product formed directly from this causes the nitrogen to be a positive ion itself, so another ammonia molecule is needed to remove an excess proton which allows the pair of electrons to be returned to the nitrogen atom forming the stable amine. As well as this an ammonium bromide salt is formed. This is needs to be done in ethanolic conditions under reflux.
Be aware that this particular reaction can continue forming multiply substituted amines. This is because the amine produced can the react with the original haloalkane reactant to form secondary and tertiary amines, as well as quaternary ammonium salts. To minimise multiple substitutions ammonia needs to be in excess.
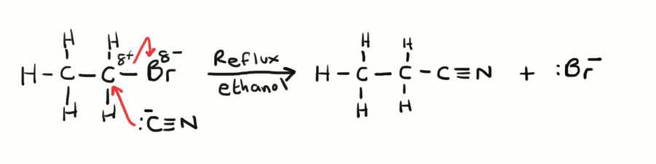
Forming Nitriles from Haloalkanes
Click image to see animation
This is also an example of nucleophilic substitution. The –CN ion donates a pair of electrons to the delta positive carbon which then forces the carbon – bromine bond to break, giving the bromine atom the electron pair in the covalent bond. For this to work it needs to be done in ethanolic conditions under reflux.