Hess’s Law
Hess law is used to find out an unknown enthalpy change. It is particularly useful for enthalpy changes that cannot be measured directly by an experiment. Hess law states that:
“The total enthalpy change for a reaction is independent of the route taken.”
Another way of saying this is not matter which route you take the enthalpy change is always the same. Let’s look at this example:
Find out the enthalpy of reaction (ΔHr) of:
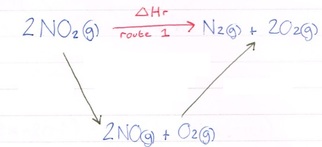
2NO2(g) --> N2(g) + 2O2(g)
If you are given these enthalpy changes:
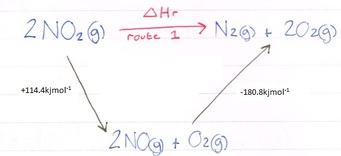
2NO2(g) --> 2NO(g) + O2(g) = 114.4kjmol-1
2NO(g) + O2(g) --> N2(g) + 2O2(g) = -180.8kjmol-1
So you have been given two known and one unknown. This is where you need to construct a Hess cycle.
Here you can see we can get from our reactant (NO2) to our products (N2 and 2O2) but by going via a different route. Hess law tells us that whatever route we take, the enthalpy change will be the same. Let’s add in our values:
We now need to add our values together ensuring that the reaction arrows are going in the same direction we are travelling, if they are not you will need to reverse them AND reverse the sign of the value. In this example they are all in the correct direction, but you will see this in our next example.
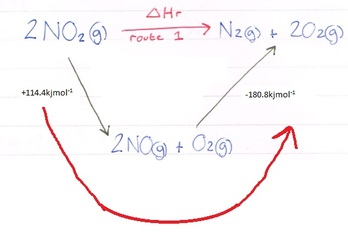
The red arrow is the route we are now taking and in this case we simply add the enthalpies together, paying attention to their signs:
114.4kjmol-1 + (-180.8kjmol-1) = -66.4kjmol-1
By far the most common questions involving Hess’s Law is those involving enthalpies or formation and combustion. Make sure you know both definitions:
Enthalpy of Combustion (ΔH⦵c): The enthalpy change when one mole of a substance is burned completely in oxygen under standard conditions.
Enthalpy of Formation (ΔH⦵f): The enthalpy change when on mole of substance is formed from its elements under standard conditions.
Don’t forget that the bus stop shaped symbol (⦵) means standard conditions which is 298K and 100kpa.
When dealing with formation values, an element will always have a value of zero by definition. It is also useful to remind yourself that a negative enthalpy value (ΔH) means exothermic, and positive means endothermic.
We will start with an enthalpy of formation question:
Calculate the enthalpy of reaction (ΔHr) for the following using the formation values given in the table:
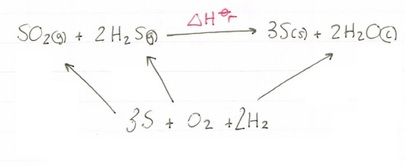
SO2(g) + 2H2S(g) --> 3S(s) + 2H2O(l)
|
ΔH⦵f [SO2(g)] |
-297kjmol-1 |
|
ΔH⦵f [H2S(g)] |
-20.2kjmol-1 |
|
ΔH⦵f [H2O(g)] |
-286kjmol-1 |
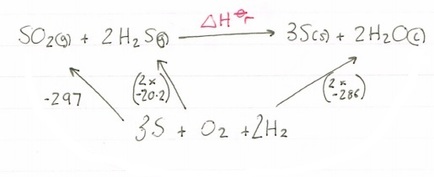
Using the formation values you have been given we need to construct our cycle. When using formation values you can just put a box at the bottom of the cycle called ‘elements’, or you can actually write down the elements used, the choice is yours:
Now we have constructed our cycle lets add in our values, paying attention to the number of moles of each one!
As you can see because we are forming one mole of SO2, we can leave it as -297, but for H2S there are two moles, so we need to multiply our value by two, and again for water, we multiply it by three. Note how there is no value for S, that is because it is an element and the formation values of elements, by definitions are zero.
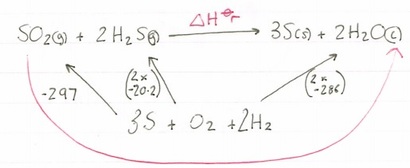
Now let’s look at the route we need to take to get to our products.
This time we have our route arrow going against some arrows so for our calculation we need to invert their signs, so -297 becomes +297 and -40.4 becomes + 40.4. Once that is done we need to add them together like before.
337.4 + (-572) = -234.6kjmol-1
Now let’s try an enthalpy of combustion question:
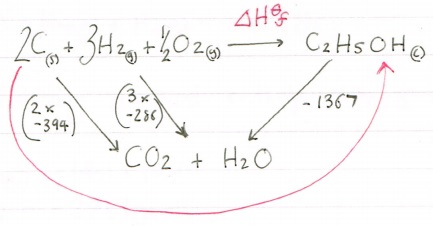
Calculate ΔH⦵f for ethanol:
C(s) + H2(g) + O2(g) --> C2H5OH(l)
|
ΔH⦵c [C2H5OH(l)] |
-1367kjmol-1 |
|
ΔH⦵c [C(s)] |
-394kjmol-1 |
|
ΔH⦵c [H2(g)] |
-286kjmol-1 |
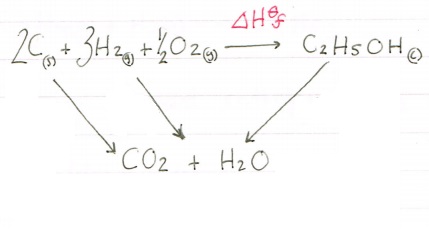
Using the combustion values you have been given we need to construct our cycle. When using combustion values you can put a box at the bottom with CO2 and H2O because these are your combustion products.
Now we have constructed our cycle lets add in our values, paying attention to the number of moles of each one!
This time we are forming the carbon dioxide and water from multiple moles of reactants so we need to be careful and ensure we multiply correctly. We need to multiply the carbon value by two and the hydrogen value by three. There is no combustion value for oxygen as you cannot react oxygen with itself.
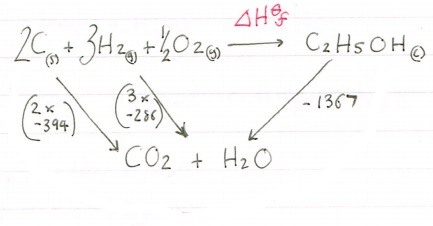
Now let’s look at the route we need to take to get to our products.
Again we have our route arrow going against an arrow so for our calculation we need to invert its sign, so -1367 becomes +1367. Once that is done we need to add them together:
-1646 + (+1367) = -279kjmol-1