Equilibrium
Many reactions around you are reversible. They can go backwards and forwards. For a chemical reaction it means that the reactants will react to make the products, but also the products will react to make the reactants. The result is that it is difficult to fully change the reactants into products! This is important in industrial processes because it would be better if the reactants reacted completely.
For example Copper Sulfate. When we heat blue hydrated copper sulfate it becomes white anhydrous copper sulfate as the water of crystallisation is removed.
CuSO4.5H2O(s) ⇌ CuSO4(s) + 5H2O(l)
If I was to add the white anhydrous copper sulfate to water, it would return to blue.
Dynamic Equilibrium
In a reversible reaction, when the concentration of reactants and products are constant, then the reaction is at equilibrium. At equilibrium, the reactants are still making the products, and the products are still reacting to make the reactants, so the reaction is called a dynamic equilibrium. The word ‘dynamic’ means ‘active’. The speed at which reactants react is called the forward rate, and the speed at which the products react is called the backward rate. It is important to remember that in a dynamic equilibrium:
- The forward rate equals the backward rate
At equilibrium the concentrations of the reactants and products may be constant, but that does not mean that the reactant concentration equals the product concentration. Really there is a fixed ratio between the reactants and products. At equilibrium the concentration of the chemicals do not change until the conditions are changed.
Le Chateliers Principle
When a reaction is at equilibrium and you try to change the amounts of the chemicals, then the position of equilibrium will shift to resist the change. This is described in Le Chatelier’s Principle which says:
“When the conditions of an equilibrium are changed then the position of equilibrium will shift to minimize the change.”
When an equilibrium shifts to make more reactants, and so less products, then you can say that “the equilibrium shifts to the left”. When an equilibrium shifts to make more products, and so less reactants, then you can say that “the equilibrium shifts to the right”.
Look at the reversible reaction that stores hydrogen fuel as methanol:
CO2(g) + 2H2(g) --> CH3OH(l) (methanol)
What will happen as the hydrogen is taken away to make energy?
The answer is that more hydrogen is made because some methanol reacts. This is because:
- At equilibrium the amounts of all three chemicals remain constant.
- When some hydrogen is removed the position of equilibrium will shift to resist the change.
- More hydrogen is made because some methanol reacts to make carbon monoxide and hydrogen.
If this equilibrium mixture is in a car fuel tank, then the energy from the car braking could be used to make electricity, which is used to make hydrogen that is pumped into the fuel tank. What will happen then? The position of equilibrium will shift to the right, to make more methanol, and so use up most of the hydrogen.
Effect of changing a concentration
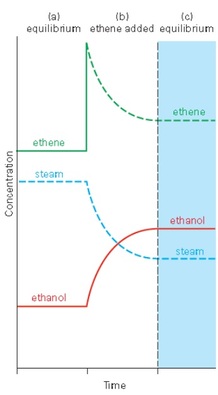
The position of equilibrium will change if the concentration of one reactants or products is changed. Here is a reaction that is used to make the common alcohol, ethanol;
ethene + steam ⇌ ethanol vapour
CH2CH2(g) + H2O(g) ⇌ CH3CH2OH(g)
Think of the mixture being at equilibrium. If the concentration of ethene is increased then:
- the position of equilibrium will shift to resist the change
- the position of equilibrium will shift to the right
- more ethanol will be made
If the concentration of water is decreased then:
- the position of equilibrium will shift to resist the change
- the position of equilibrium will shift to the left
- less ethanol will be made
If the concentration of ethanol is decreased then
- the position of equilibrium will shift to resist the change
- the position of equilibrium will shift to the right
- more ethanol will be made
Changing Pressure
Which way will the position of equilibrium shift when you change the pressure? The equilibrium will shift to resist the change. Take the previous example:
ethene + steam ⇌ ethanol vapour
CH2CH2(g) + H2O(g) ⇌ CH3CH2OH(g)
If this mixture reaction is at equilibrium, and the pressure is increased, then which way would the position of equilibrium shift?
- There are two gas molecules on the left and one gas molecule on the right.
- Two gas molecules would produce more pressure than one gas molecule in the same box.
- To reduce the pressure, the number of gas molecules must get smaller.
- In this example, an increase in pressure would shift the position of equilibrium to the right, to produce more ethanol, and fewer ethene and water molecules.
It is important to remember that when the pressure is increased the position of equilibrium will shift in the direction of the side with the fewer gas molecules.
Changing Temperature
Which way does the position of equilibrium shift when you change the temperature? The position of equilibrium will shift to resist the change:
ethene + steam ⇌ ethanol vapour
CH2CH2(g) + H2O(g) ⇌ CH3CH2OH(g) ΔH = -45kjmol-1
This reaction is exothermic (it has a negative enthalpy value) and if this reaction mixture was at equilibrium, what would happen if the temperature was increased?
- The reaction is labelled as an exothermic reaction, which means the forward reaction is exothermic.
- The backward reaction is endothermic.
- If the temperature is increased then the position of equilibrium will shift to resist the change and so cool the mixture.
- The endothermic reaction is favoured, which is the backward reaction.
- In this example, the position of equilibrium shifts to the left.
It is important to remember that if the temperature increases then the position of equilibrium will shift in the endothermic direction.
Adding a Catalyst
When adding a catalyst the reaction rate does increase, the amount of product is unchanged. This is because the catalyst will speed up the reaction in both the forward AND the backwards directions. It is therefore important to remember that in an equilibrium, catalysts have no effect on the position of equilibrium.