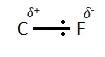Electronegativity
Electronegativity is a measure of the ability of an atom to attract a bonding pair of electrons and the values usually given from the Pauling scale (for A-level anyways). Fluorine, which is the most electronegative atom, is given a value of 4.0, and values go all the way down to caesium and francium which are the least electronegative at 0.7. However you must but be aware that the values are all relative, so a difference of 0.5 between 3.5-3.0 is not the same as a difference of 0.5 between 2.5 – 2.0.
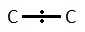
If we take a carbon – carbon bond:
The line represents the covalent bond and the dots the lone pair of electrons. This is an average pictures because the electrons are always moving but “on average” the electron pair would be found within middle of the covalent bond because carbon atoms both have the same electronegativity values and this is therefore a non-polar bond.
A high electronegativity value means that the element attracts the bonding pair of electrons much more readily than an element with a very low electronegativity value. A good example would be a carbon-fluorine bond. Fluorine has an electronegativity value of 4.0 and Carbon has an electronegativity value of 2.5. This is telling us that Fluorine is much more electronegative than Carbon.
As you can see this means that the electron pair in the covalent bond is (on average) much closer to the fluorine atom compared to the carbon atom. This is a polar bond. To show that a bond is polar we can add delta charges. If the electron is much closer to one of the atoms then it will be given a delta negative charge and if it is further away it is given a delta positive charge. The delta symbol is □.
You can explain the idea of electronegativity with dot and cross diagrams and a concept called effective nuclear charge.
Effective Nuclear Charge
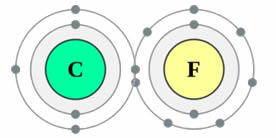
Both carbon and fluorine are in period two, so this means they both have a full inner orbital with two electrons. The carbon atom has six protons in the nucleus and the fluorine atoms has nine. There are two electrons (in the 1s orbital) on both carbon and fluorine “shielding” the outer electrons from the full force of the nucleus and due to both atoms being approximately the same size (both period two) then it is this shielding affect and proton difference that is causing the electronegativity difference.
One way to look at this is to remove these two inner electrons from the total nuclear pull (think of these two inner electrons cancelling out one proton each) and calculating an “effective nuclear charge”. So we can think of fluorine which has 9 protons, having two cancelled out from the shielding affect and therefore it has an effective nuclear charge of +7, whereas carbon has six electrons in total and after two being cancelled out by shielding from the 1s would then have an effective nuclear charge of +4.
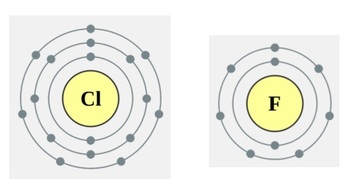
This method is a good way at looking at why the electronegativity increases across a period, but what about down a group? Why is chlorine NOT as electronegative as Fluorine? Well this is a bit simpler, the outer electrons are further away from the nucleus! If you work out the effective nuclear charge of both fluorine and chlorine, then you will find they are both +7:
Fluorine Protons = 9
Shielding electrons, in this case 1s = 2
9 – 2 = +7
Chlorine protons = 17
Shielding electrons, in this case 1s AND 2s AND 2p = 10
17 – 10 = +7
You can also clearly see that the outer electrons on chlorine are further away from the nucleus, which means they experience less pull and hence its electronegativity value is lower.
Polar Bonds and Polar molecules
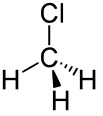
I have spoken about electronegativity and mentioned polar bonds. To be precise a polar bond is a bond with an uneven distribution of charge which means that if the bond has an electronegativity difference then the bond is also polar. However just because a molecule has a polar bond, does NOT mean it is a polar molecule (confusing huh?). It is all in the description “uneven distribution of charge”. Take chloromethane:
The carbon – chlorine bond is polar because of the electronegativity difference between the two atoms. This is ALSO a polar molecule.
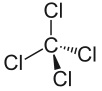
But take tetrachloromethane:
The carbon – chlorine bonds are all polar because of the electronegativity difference between the two atoms BUT this is NOT a polar molecule. Because all of the four bonds are polar there is now not an uneven distribution of charge across the molecule, and it is therefore not polar.
An easy way of determining if a molecule is polar (liquids only) is to take a charged polythene rod and pour a stream of the liquid. If it is polar then the liquid stream will bend, if it is non polar it will not. You can try this with water (which is a polar molecule) and it will bend, however a molecule such as cyclohexane does not.