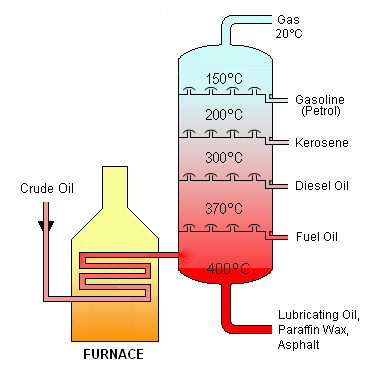
Crude Oil
Currently the world’s main source of organic chemicals, it was produced over millions of years by the breakdown of plant and animal remains at high pressure and temperatures deep below the sea. Because it was made so long ago it is known as a fossil fuel and is not renewable.
Crude oil is a mixture mostly of alkanes, both unbranched and branched. Crude oils from different sources have different compositions. It also small amounts of other compounds and some of these contain sulfur. These produce sulfur dioxide, SO2, when they are burned. This is one of the causes of acid rain as it reacts with oxygen and water in the atmosphere to form sulfuric acid.
Fractional Distillation of Crude Oil
To convert crude oil into useful products we must separate this mixture. We can do this by heating and then collecting the fractions. This can be done because each of the fractions have a different boiling point.
Fractionating Tower
- The crude oil is first heated in a furnace so that it vaporises.
- The vapours pass into a tower that is cooler at the top than the bottom.
- They pass up the tower via a series of trays containing bubble caps until they arrive at a tray that is sufficiently cool (at a lower temperature than their boiling point). Then they condense to a liquid.
- The mixture of liquids that condenses on each tray is piped off.
- The shorter chain hydrocarbons condense in the trays nearer to the top of the tower, where it is cooler, because they have lower boiling points.
- The thick residue that collects at the base of the tower is called bitumen or tar and is used for road surfacing.
Cracking
Cracking is needed because when fractional distillation of crude oil occurs, you get a very high percentage of long chain hydrocarbons. So the results of cracking are:
- Shorter, more useful chains are produced
- Some of the products produced are alkenes. These can be converted to other compounds very easily and are therefore very useful.
There are two main types of cracking:
Thermal Cracking
This reaction involves heating alkanes to a high temperature, under high pressure. Carbon-carbon bonds break homolytically and two shorter chain are produced each ending in an unpaired electron. As there are not enough hydrogens to produce two alkanes, one of the new chains must have a carbon-carbon double bond.
In thermal cracking, any number of carbon-carbon bonds can break, and anywhere on the chain.
Catalytic Cracking
This kind of cracking takes place at a much lower temperature (approx. 800K) and uses a zeolite catalyst which consists of silicon dioxide and aluminium oxide. Zeolites, as well as being acidic, have a honeycomb structure which has an enormous surface area. This form of cracking is used to produce hydrocarbons such as motor fuels and aromatic compounds.
The products of catalytic cracking are mainly gases, which means chain lengths of less than five. The mixture also decolourises bromine showing that it contains alkenes as well. A number of other processes are carried out on the products of fractional distillation and cracking, which aim to improve the quality of the products, such as motor fuels.
Isomerisation
Straight chain alkanes make relatively poor motor fuels. Straight chain alkanes burn too quickly causing what is often called “knocking” in the engine. Branched chain alkanes burn more slowly and steadily promoting efficient combustion.
To produce branched chain alkanes you heat the straight chains under pressure with a platinum catalyst.
Reformation
In reformation we heat straight chain alkanes under pressure with a catalyst of platinum and aluminium, this removes hydrogen in steps ultimately ending up with cyclic hydrocarbons (rings).
Crude oil and the Environment
All hydrocarbon fuels produce polluting products when they burn. These include:
- Carbon monoxide –Toxic gas
- Unburnt hydrocarbons –These may form photochemical smog
- Carbon dioxide –A greenhouse gas
- Nitrogen dioxides –Contribute to acid rain
- Nitrogen dioxides –Contribute to acid rain
- Sulfur dioxides –Contribute to acid rain
As you are no doubt award, crude oil is a fossil fuel, meaning that it takes millions of years to be produced from plant and animal matter decay and hence cannot be renewed in the short term, so new alternatives source of energy must be found.
Biofuels
These are fuels that are obtained from fast growing plants so they are renewable. Some biofuels can be used as they are, for example straw or wood can be burnt directly. Other crops such as sugar or rape seed oil need chemical treatment to turn them into useable sources of energy.
Ethanol
Ethanol (CH3CH2OH), when made from the fermentation of sugar and other carbohydrates obtained from plants, is a biofuel. It burns in oxygen to form carbon dioxide and water.
CH3CH2OH(l) + 3O2(g) --> 2CO2(g) + 3H2O(l) ΔH -1367 kJ mol-1
Ethanol has an energy density of about 30 kJ per gram, significantly less than alkane fuels (around 50 kJ per gram). However, with some modifications, normal car engines can run on pure ethanol, or ethanol/petrol mixtures. This is quite prevalent in some countries and one of the benefits is that it reduces the need to import crude oil. Sadly, it does have one major drawback, and that is the need for huge areas of land to grow the correct crops to produce the ethanol. This often means either reducing the number of edible crops grown, or more often destroying forests and other areas of nature.