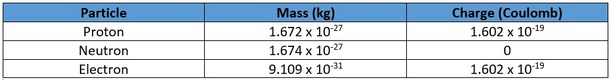
Atoms are made from three sub-atomic particles. These are called protons, neutrons, and electrons. The table shows the mass and electric charge of each sub-atomic particle.
|
Any atom has an equal number of protons and electrons. This means that atoms are neutral overall. For example, fluorine atoms each have nine protons and nine electrons. The nine positive charges carried by the protons in the nucleus are balanced by the nine negative charges carried by the electrons. Because neutrons are neutral, there can be any number of neutrons in the nucleus without making any difference to the overall charge of the atom.
|
The atomic number of an atom is the number of protons in its nucleus. It is also called the proton number. The atomic number is shown by the symbol Z. Every element has its own unique atomic number. All the atoms of a particular element have the same atomic number.
The mass number of an atom is the number of sub-atomic particles in its nucleus. The mass number is shown by the symbol A. It is the number of neutrons added to the number of protons. The full symbol for an atom is written like this:
The mass number of an atom is the number of sub-atomic particles in its nucleus. The mass number is shown by the symbol A. It is the number of neutrons added to the number of protons. The full symbol for an atom is written like this:
The atomic number is shown as a subscript and the mass number as a superscript. You can work out the number of neutrons in an atom by subtracting the atomic number from the mass number. So for \(F\begin{array}{*{20}{c}}{19}\\9\end{array}\) the number of neutrons is 19 - 9 = 10. The number of protons (and the number of electrons) in the atom is 9.
Isotopes
Isotopes are atoms that have the same number of protons but different numbers of neutrons in their nuclei. For example, \(Cl\begin{array}{*{20}{c}}{35}\\{17}\end{array}\) and \(Cl\begin{array}{*{20}{c}}{37}\\{17}\end{array}\) are both isotopes of chlorine. Their atoms each contain 17 protons. But every \(Cl\begin{array}{*{20}{c}}{35}\\{17}\end{array}\) atom has 35 - 17 = 18 neutrons, and every \(Cl\begin{array}{*{20}{c}}{37}\\{17}\end{array}\) atom has 37 - 17 = 20 neutrons. The different isotopes of an element are chemically identical to each other because they contain the same number of protons and electrons.