Aromatic Mechanisms
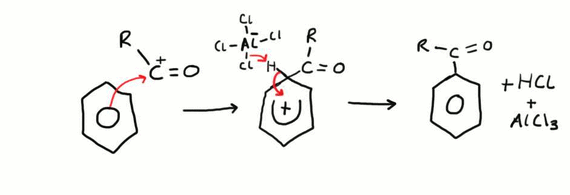
Friedel-Crafts Acylation
Click image to see animation
This is an example of an electrophilic substitution reaction. Firstly, the electrophile is generated by the AlCl3 catalyst removing the chlorine atom from the acyl chloride. The newly generated RCO + electrophile is then attacked by the electron dense ring and a bond is formed. Next an aluminium – chlorine bond breaks and the electron pair is used to gain a hydrogen ion from the benzene ring, and the electron pair originally between the carbon and hydrogen bond is then transferred back into the ring to maintain the electron density. This reaction is done under reflux conditions and in a non-aqueous environment.
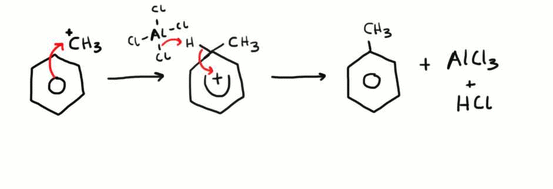
Friedel-Crafts Alkylation
Click image to see animation
This is an example of an electrophilic substitution reaction. Firstly, the electrophile is generated by the AlCl3 catalyst removing the chlorine atom from a halogenoalkane (in this case chloromethane). The newly generated CH3+ electrophile is then attacked by the electron dense ring and a bond is formed. Next an aluminium – chlorine bond breaks and the electron pair is used to gain a hydrogen ion from the benzene ring, and the electron pair originally between the carbon and hydrogen bond is then transferred back into the ring to maintain the electron density. This reaction is done under reflux conditions and in a non-aqueous environment.
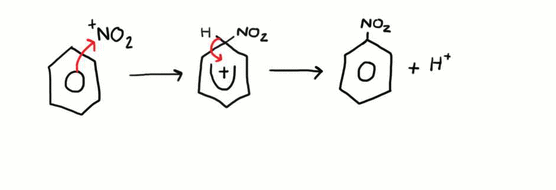
Nitration of Benzene
Click image to see animation
This is an example of an electrophilic substitution reaction. Firstly, the electrophile is generated by the reaction between sulfuric acid and nitric acid. The sulfuric acid will ultimately act as a catalyst as it will be regenerated. The reaction is as follows:
HNO3 + 2H2SO4 --> NO2+ + 2HSO4- + H3O+
The newly formed NO2+ electrophile is then attacked by the electron dense ring and a bond is formed. The electron pair between the original carbon – hydrogen bond on the ring is then donated back into the ring to maintain the original electron density and a hydrogen ion is lost. This reaction needs to be kept below 55oC otherwise multiple substitutions can occur.
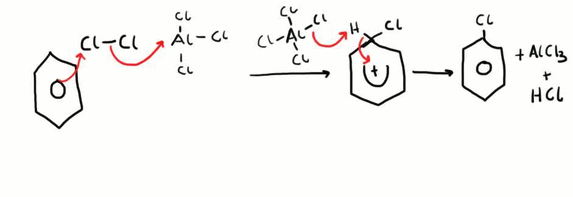
Halogenation of Benzene
Click image to see animation
This is an example of an electrophilic substitution reaction. Firstly, the electrophile is generated by the reaction between chlorine and aluminium chloride. The benzene ring approaches the chlorine molecule and the electron pair in the chlorine – chlorine bond is repelled by the electron dense benzene ring, thus forming a temporary dipole. The delta positive end of the molecule is then able to be attacked by the ring and a bond is formed, the other chlorine atom forms a bond with the AlCl3 forming AlCl4+. Next an aluminium – chlorine bond breaks and the electron pair is used to gain a hydrogen ion from the benzene ring, and the electron pair originally between the carbon and hydrogen bond is then transferred back into the ring to maintain the electron density.
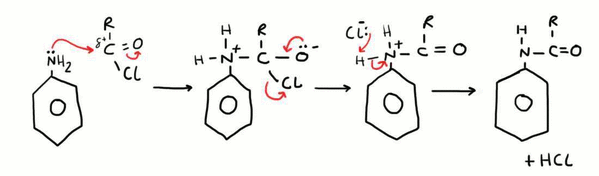
Formation of N-substituted Amines from Phenylamine